Medical research safety is paramount in ensuring the well-being of participants enrolled in clinical studies. The integrity of research relies heavily on meticulous oversight, particularly from Institutional Review Boards (IRBs), which play a vital role in safeguarding patient safety in research initiatives. When there are disruptions in research funding, such as the recent federal cuts experienced at Harvard, the oversight mechanism that protects patients can be severely compromised. Research oversight, often dependent on federal funding medical research, ensures compliance with ethical and legal standards, ultimately protecting the rights and welfare of participants. Without robust funding, the potential impacts of research oversight deficiencies can undermine public trust and hinder advancement in medical treatments.
In the realm of clinical trials, the importance of ensuring participant safety cannot be overstated. Various mechanisms, including ethical reviews conducted by oversight bodies like Institutional Review Boards (IRBs), are fundamental to maintaining high standards of care in research endeavors. These IRBs, which evaluate the protocols and potential risks involved, are critical in fostering an environment where research can flourish ethically and responsibly. However, recent funding reductions have raised concerns about the sustainability of these oversight efforts, potentially jeopardizing patient welfare and the efficacy of ongoing studies. As the landscape of medical inquiry evolves, we must prioritize resources that support patient safety and uphold research integrity.
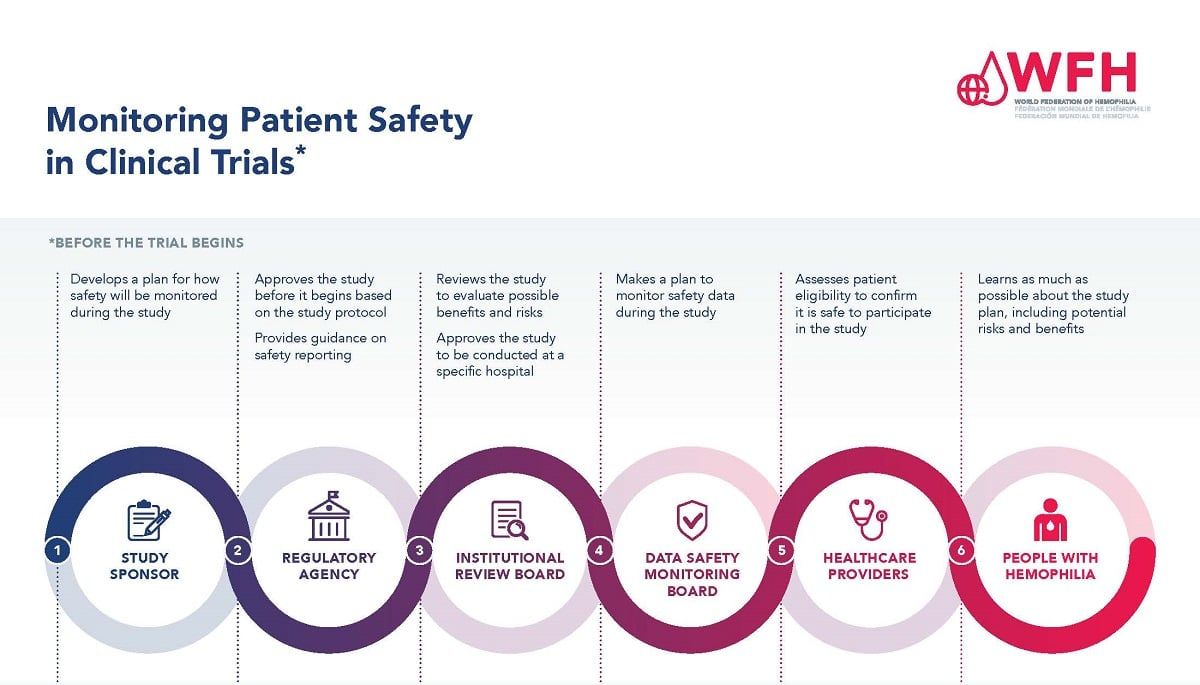
The Critical Role of Medical Research Safety in Clinical Trials
Medical research safety is paramount in clinical trials, primarily because these studies involve real patients who are subjected to new treatments, medications, or procedures. Understanding the risks and benefits is crucial; thus, robust processes need to be instituted to safeguard participants. Institutional Review Boards (IRBs) serve this purpose by reviewing research proposals to mitigate risks and ensure informed consent is appropriately gathered. When funding for these oversight mechanisms is interrupted, as seen with recent federal funding cuts, the integrity of patient safety in research comes into question.
Furthermore, the ripple effects from funding cuts can have dire consequences beyond the immediate halting of studies. With fewer resources allocated for research oversight, the ability of IRBs to conduct thorough reviews diminishes. This leads to a compromised environment for patient safety, increasing the likelihood of ethical violations and potential harm to participants. Thus, maintaining a steady flow of federal funding is vital not only for the advancement of science but also for ensuring the safety and rights of individuals involved in medical research.
Impact of Funding Cuts on Patient Safety and Research Oversight
The impact of funding cuts on patient safety cannot be overstated, especially in the context of medical research where oversight is critical. Federal funding cuts, like the recent stop-work orders affecting institutions such as Harvard, lead to the suspension of essential research projects that have direct implications for patient safety. Without adequate funding, the ability of IRBs to function effectively is curtailed, jeopardizing their capacity to monitor ongoing studies and enforce compliance with ethical standards. This lack of oversight can lead to increased risk for patients who participate in trials without adequate protection and support.
Moreover, the challenges posed by funding cuts extend beyond individual trials and affect the broader landscape of medical research. When robust research oversight systems are compromised, mistrust builds within communities, impacting patient recruitment for clinical trials. Public confidence in research efforts diminishes, stifling innovation and the development of new therapies that could benefit collective health. Therefore, ensuring the financial stability of research oversight frameworks is crucial for protecting patient safety and ensuring the continued progress of medical research.
Institutional Review Board (IRB) Functions and Responsibilities
Institutional Review Boards (IRBs) play a pivotal role in upholding the ethical standards of clinical research. Their primary responsibility is to review research proposals to ensure they comply with regulations designed to protect the welfare of participants. By assessing the ethical implications of proposed studies, IRBs can identify potential risks and ensure that appropriate measures are taken to mitigate those risks. For instance, IRBs evaluate the study design, participant recruitment strategies, and informed consent processes, safeguarding the interests of individuals involved in research.
Additionally, IRBs are tasked with monitoring ongoing research to ensure compliance throughout the study lifecycle. This ongoing oversight is vital for quickly addressing emerging ethical concerns and making necessary adjustments. IRBs also serve as a resource for researchers, providing necessary training and support to help them navigate the ethical complexities of their projects. This education is crucial for fostering a culture of compliance and ethical engagement in research, ultimately ensuring patient safety amid ongoing clinical trials.
The Interplay Between Federal Funding and Patient Safety
Federal funding is essential for supporting the intricate network of research oversight that directly influences patient safety. When grants and contracts are secure, research institutions can allocate adequate resources toward their IRB operations, allowing for comprehensive reviews and continuous monitoring of clinical trials. However, with the disruption caused by funding cuts, such as the recent order affecting Harvard, the flow of resources needed to maintain oversight becomes compromised. This creates a scenario where patient safety may increasingly be at risk, highlighting the need for reliable funding sources to support these vital systems.
Moreover, the impact of federal funding on patient safety is multifaceted. Increased funding can also lead to more rigorous training for IRB members and researchers, enhancing their capability to protect participants’ rights and well-being. Conversely, funding cuts can lead to a reduction in the workforce dedicated to overseeing research, limiting the capacity to perform essential audits and compliance checks. As a result, the health and safety of patients who participate in medical research hinge significantly on the stability of federal funding.
Exploring Patient Rights in Medical Research
Ensuring the rights of patients who participate in medical research is a responsibility shared by researchers, institutions, and IRBs. Participants must be fully informed of the study’s purpose, procedures, potential risks, and benefits before giving consent. This informed consent process is a cornerstone of ethical research practice, reinforcing the autonomy of patients and their right to make decisions about their own healthcare. When research oversight mechanisms like IRBs are adequately funded and operational, the protection of these rights is further enhanced, allowing for transparent dialogue between researchers and participants.
In light of recent funding cuts, however, the enforcement of patient rights may face significant challenges. Without the necessary resources, IRBs might struggle to conduct thorough reviews that ensure participant well-being and ethical compliance, which could lead to breaches of trust and potential violations of rights. This disruption not only affects individual patients but also has broader implications for public confidence in the research community. To truly uphold patient rights, it is imperative to sustain robust funding strategies that bolster the ethical conduct of medical research.
The Historical Context of Human Research Protections
The establishment of human research protections can be traced back to several historical injustices that highlighted the dire need for oversight in medical studies. Events such as the Tuskegee Syphilis Study, where African American men were denied treatment to observe the progression of untreated syphilis, underscored the importance of ethical standards in research. These tragic incidents led to the creation of IRBs and stringent regulations to protect participants from similar abuses in the future. Understanding this historical context is crucial for appreciating the role of oversight today.
Furthermore, the evolution of research protections continues to respond to challenges that arise in clinical trials. Notable adjustments in regulations often happen in direct response to past misconduct, reinforcing the commitment to ethical oversight and patient safety. For instance, rigorous guidelines have emerged to provide safeguards against exploitation in research, ensuring that informed consent is genuinely informed. This continual evolution helps maintain a vigilant and ethical approach to clinical research, which is critical for restoring and maintaining public trust in the medical research community.
Building Public Trust in Medical Research Amid Funding Challenges
Public trust is an essential component of successful medical research, fostering participation and collaboration between researchers and communities. When trust falters, as it often does in the wake of funding cuts and disrupted oversight mechanisms, the willingness of individuals to participate in clinical trials diminishes. As a result, the very innovations that could drive healthcare improvements become stifled, ultimately affecting patient outcomes. Consequently, it is crucial for research institutions to actively engage with the communities they serve to rebuild trust during periods of funding uncertainty.
Additionally, transparency in research practices plays a significant role in cultivating public confidence. Open communication regarding funding sources, research processes, and oversight mechanisms reassures potential participants that their safety is prioritized. As research institutions navigate funding cuts, maintaining an open dialogue with the public can help bridge the gap created by financial disruptions. By demonstrating their commitment to ethical standards and patient safety, institutions can foster a collaborative environment that encourages participation in research endeavors.
Future Directions for Patient Safety in Medical Research
Looking ahead, the future of patient safety in medical research relies heavily on strategic investments in research oversight and ethical practices. Ensuring sustainable funding for IRBs and related oversight committees is essential to maintaining a high standard of care for participants. As the healthcare landscape evolves, so too must the frameworks designed to protect patient welfare, adapting to new challenges and technologies. By prioritizing the financial viability of research oversight systems, we can safeguard patient safety while fostering innovation in clinical research.
Moreover, proactive measures can enhance the overall ethical conduct of medical research. This includes ongoing education and training for researchers, IRB members, and institutional staff to reinforce their understanding of ethical obligations. Encouraging collaborative research environments that prioritize participant safety can also lead to more innovative, effective medical solutions. Aligning future research efforts with ethical oversight will not only protect patients but can also bolster the public’s trust in medical research, ensuring a more collaborative approach moving forward.
Navigating Ethical Dilemmas in Clinical Trials
Navigating ethical dilemmas in clinical trials presents significant challenges for researchers and IRBs alike. Many studies involve compromises between advancing medical knowledge and ensuring the safety and rights of participants. Ethical dilemmas can arise from various sources, including unexpected adverse events, conflicts of interest, or uncertainties around informed consent. When faced with such dilemmas, it is crucial for research institutions to employ robust ethical frameworks and engage in systematic reviews to evaluate the risks and benefits of continuing or modifying study protocols.
Moreover, the role of IRBs becomes even more critical during times of ethical ambiguity. These boards must not only serve as watchdogs for compliance and ethical conduct but also assist in fostering an environment where open dialogue about risks is encouraged. This engagement ensures that all voices — from researchers to participants — are heard when making critical decisions about study integrity. Ultimately, the ability to address ethical dilemmas effectively is dependent on maintaining a strong foundation of funding and oversight that supports the essential work of IRBs.
The Future Landscape of Funding in Medical Research
The future of funding for medical research hinges on navigating the intertwined relationship between financial support and research outcomes. With ongoing challenges such as budget constraints and political changes, the landscape is rapidly evolving, necessitating a clear understanding of funding mechanisms and their implications for research oversight. Institutions must advocate for sustained federal and private funding to ensure robust oversight and ethical compliance, which are crucial for patient safety in clinical trials.
Additionally, there is an increasing need for alternative funding models to support research initiatives, particularly within the realms of patient-focused studies. Engaging stakeholders, including community members and private sectors, to contribute to research funding can foster a sense of shared ownership and accountability in research endeavors. By creating diverse funding streams, research institutions can mitigate the risks associated with funding cuts, ensuring that patient safety remains a priority in medical research.
Frequently Asked Questions
How does patient safety in research relate to the role of Institutional Review Boards (IRBs)?
Patient safety in medical research is fundamentally linked to the role of Institutional Review Boards (IRBs). IRBs are responsible for reviewing and approving research proposals to ensure that the rights, welfare, and safety of research participants are protected. They assess the study designs, informed consent processes, and potential risks to participants, making them a critical component of research oversight.
What are the impacts of federal funding cuts on research oversight and patient safety?
Federal funding cuts significantly impact research oversight and patient safety. When funding is reduced or stopped, vital programs like SMART IRB, which facilitates the review and oversight of studies across multiple sites, struggle to operate effectively. This can lead to delays in ongoing studies, increased risk to participants, and a potential decline in the overall quality of research conducted, ultimately jeopardizing patient safety.
What is the importance of federal funding in ensuring patient safety in medical research?
Federal funding plays a crucial role in ensuring patient safety in medical research by providing the necessary resources for robust IRB oversight and compliance with ethical standards. Funding supports training for research staff, operational management of IRBs, and the implementation of safety protocols that protect participants throughout the research process.
How do IRBs contribute to ensuring patient safety in clinical trials funded by the NIH?
IRBs contribute to patient safety in NIH-funded clinical trials by conducting thorough reviews of research protocols, evaluating the ethical implications, and ensuring that appropriate measures are in place to mitigate risks to participants. This includes ongoing monitoring of trials, assessment of adverse events, and safeguarding informed consent.
What happens to patient safety in research studies when there is a halt in funding?
A halt in funding can severely compromise patient safety in research studies. It can disrupt the operations of IRBs, delay the initiation of necessary safety protocols, and impede the ability to monitor research effectively. This can lead to increased risks for participants, loss of trust in the research community, and potential harm to the public’s health.
How do changes in IRB policies affect patient safety in medical research?
Changes in IRB policies can directly affect patient safety in medical research by altering the requirements for study reviews and participant oversight. For example, the implementation of a single IRB for multisite studies can streamline processes, potentially enhancing safety by facilitating coordinated oversight, but it must be carefully managed to ensure that standards are met across all sites.
What role do community trust and engagement play in maintaining research safety?
Community trust and engagement are essential for maintaining research safety. When communities feel involved and informed about research studies, it enhances transparency and ethical conduct. Engaging with communities fosters cooperation, leading to better participant recruitment, adherence to study protocols, and overall safety for participants involved in medical research.
How can researchers ensure compliance with patient safety regulations in healthcare studies?
Researchers can ensure compliance with patient safety regulations in healthcare studies by actively engaging with IRBs, adhering to established protocols, conducting thorough training on ethical research practices, and continuously monitoring and reporting any adverse events. Staying informed about regulatory changes also helps maintain compliance and safeguard the welfare of research participants.
| Key Point | Details |
|---|---|
| Funding Cuts | The Trump administration’s freeze of over $2 billion in federal research grants to Harvard disrupts patient safety efforts in medical research. |
| Institutional Review Board (IRB) Role | IRBs ensure the protection of human participants through oversight and compliance with policies and regulations. |
| Impact of SMART IRB | SMART IRB facilitates collaboration among institutions, but the stop-work order halts new clinical sites and delays research. |
| Historical Context | Past medical ethics violations highlight the importance of careful monitoring and oversight in research. |
| Concerns for Participants | IRBs ensure participants understand risks and benefits, fostering informed consent and ethical participation. |
| Community Trust | Funding cuts increase community skepticism and mistrust, harming the relationship between researchers and participants. |
Summary
Medical research safety is critically impacted by funding cuts, as it endangers the rights and welfare of participants involved in studies. The halt in federal funding disrupts oversight mechanisms essential for safeguarding individuals in research projects. Without continued support, there is a risk of eroding public trust in the research process, ultimately jeopardizing the future of medical advancements and patient safety.