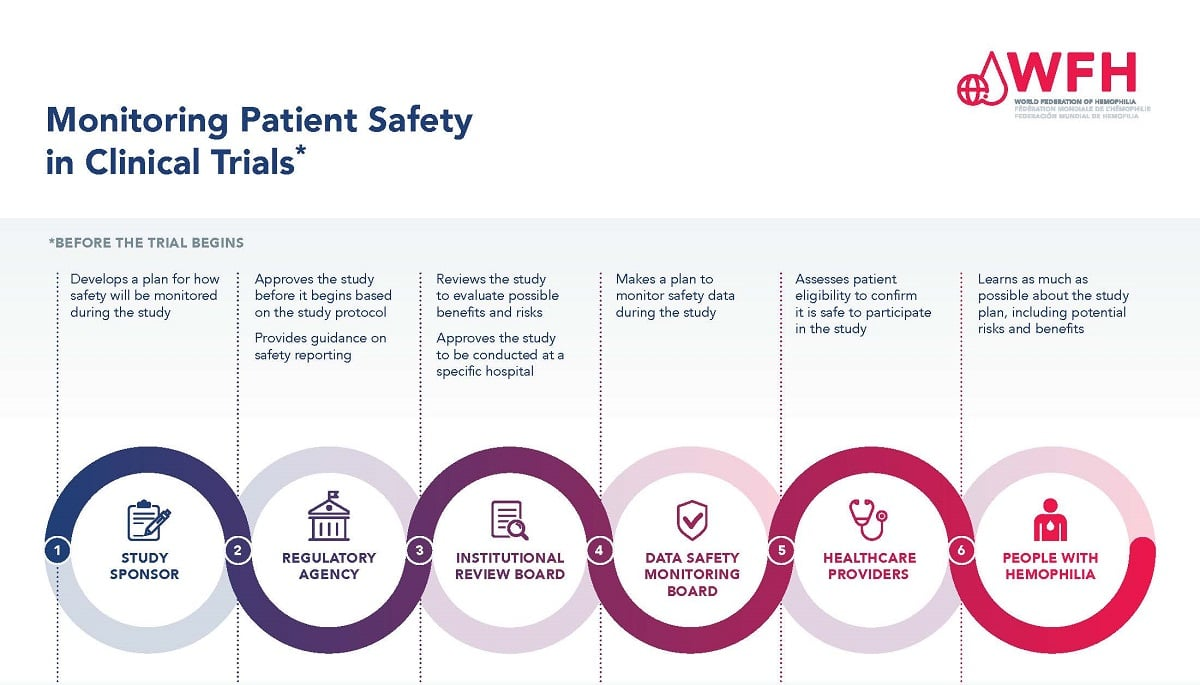
Patient safety in medical research is a paramount concern that underscores the ethical framework guiding clinical trials and studies. As more than $2 billion in federal research grants face cutbacks, the integrity of research participant protection is jeopardized, hampering the vital oversight mandated by institutional review boards (IRBs). IRBs play a crucial role in ensuring that medical research aligns with accepted medical research ethics, mitigating risks, and safeguarding participants’ rights throughout the research process. The interplay between clinical trial safety and funding supports the essential infrastructure that allows researchers to conduct studies without compromising participant welfare. In this challenging landscape, the commitment to patient safety remains crucial as the medical research community navigates the impacts of budget cuts and strives to uphold high standards for human subjects involved in research.
Ensuring the well-being of individuals involved in clinical studies is essential for ethical medical investigation. The responsibility for research participant welfare lies within frameworks established to govern medical inquiries, focusing on maintaining ethical practices in health-related studies. With increasing challenges, including financial restraints, the oversight by independent review boards becomes even more critical in evaluating research proposals. This system not only serves to provide IRB oversight but also guarantees that ethical standards are upheld, contributing significantly to the safety of subjects in research endeavors. As funding concerns mount, the healthcare community must prioritize the protection of study participants to sustain trust and progress in medical innovation.
The Importance of Patient Safety in Medical Research
Patient safety is a cornerstone of ethical medical research, ensuring that individuals who participate in studies are not only informed but also protected from undue harm. With the involvement of Institutional Review Boards (IRBs), researchers must adhere to stringent protocols that assess the risks and benefits of clinical trials. This oversight is crucial for maintaining the integrity of medical research while safeguarding the well-being of participants. When funding for research is cut, as seen in recent events, the systems that uphold these standards are jeopardized, potentially compromising patient safety.
In times of funding disruptions, the consequences for patient safety can be severe. Ongoing trials may halt, delaying access to potentially life-saving treatments while leaving participants in limbo. Specifically, IRBs play an essential role in reviewing and approving studies by assessing methodologies, which involve understanding the risks associated with interventions. If there is reduced oversight due to funding cuts, the ethical conduct of research could be significantly undermined, leading to avoidable risks for patients who depend on these clinical trials for novel therapies.
How Funding Cuts Affect Research Participant Protection
Funding is the backbone of any research initiative, especially when it comes to projects involving human subjects. The cuts to federal research grants not only stall ongoing studies but also risk the fundamental protections for research participants. When financial resources are stripped away, the ability to follow rigorous ethical standards diminishes, which can lead to lapses in oversight by IRBs. Without adequate funding, there may be insufficient support to ensure that participant rights are upheld, putting vulnerable populations at an elevated risk.
Moreover, decreased funding can exacerbate issues of trust between researchers and communities, particularly among marginalized groups who may feel their rights and safety are not being prioritized. Historically, breaches of trust in medical research—such as unethical experiments conducted without informed consent—highlight the critical need for robust oversight mechanisms like IRBs. When these protections are weakened due to budget constraints, it creates an environment where participants might be more susceptible to exploitation or harm, undermining the progress made in patient protection.
The Role of IRB Oversight in Ethical Medical Research Initiatives and Patient Safety
Institutional Review Boards (IRBs) are essential for ethical oversight in medical research, overseeing the safety and rights of study participants. These boards evaluate research protocols to ensure compliance with ethical standards and safety procedures, which include informed consent and risk assessments. IRB oversight not only protects participants but also fosters public confidence in scientific inquiry, ensuring that ethical considerations remain paramount. As funding conditions fluctuate, the IRB’s ability to enforce rigorous standards may also be compromised, reflecting a broader concern for the ethical conduct of research.
Furthermore, the reliance on a single IRB for multisite studies has streamlined research efforts but also stresses the importance of adequate funding to maintain these essential systems. A reduction in resources could lead to delays in approvals, affecting collaboration among multiple institutions while putting patient safety at risk. Without the necessary financial support, the vigor of ethical oversight can diminish swiftly, creating situations that could lead to ethical violations and compromise patient safety across various research settings.
The Historical Context of Ethics in Medical Research
Understanding the ethical landscape of medical research requires a look back at historical precedents that shaped modern IRB practices. Infamous cases of unethical research, such as the Tuskegee Syphilis Study, illustrate the dire need for informed consent and independent oversight. Such events catalyzed the establishment of IRBs to provide protective measures for research participants, ensuring that their safety and rights remain a priority. Today, the principles established in response to these historical injustices inform the ethical framework governing contemporary medical research practices.
As we critically examine the evolution of medical research ethics, it becomes evident that the success of these protective measures relies on sustained support and funding for IRB operations. Recent funding cuts threaten not only current research projects, but the foundational principles established to safeguard participants. The loss of vital resources can erode the ethical standards that have taken decades to develop, potentially leading to a regression in how research is conducted and a renewed risk to patient safety.
The Role of Federal Funding in Ensuring Clinical Trial Safety
Federal funding plays a pivotal role in ensuring the safety and effectiveness of clinical trials. By providing the necessary resources for monitoring and oversight, funding allows IRBs and research ethical committees to operate effectively. This financial backing is essential for conducting rigorous reviews that assess safety protocols, participant consent, and risk management strategies in clinical trials. When such funding is cut, the integrity of these reviews may be compromised, leading to increased risks for participants involved in studies.
In addition to supporting IRB functions, federal funding promotes innovations that can lead to safer and more effective healthcare solutions. Reductions in research grants can stall advancements in medical technology and treatment methodologies, which ultimately diminishes the potential for improving patient safety in the long term. The ripple effects of funding cuts extend beyond immediate research stoppages; they threaten the overall progress in ensuring clinical trial safety and the protection of participants, who deserve to participate in ethically sound and monitored research.
Navigating the Future of Medical Research Ethics
The landscape of medical research is currently undergoing significant changes due to broader financial constraints and ethical challenges. As IRBs grapple with budget cuts, the imperative to uphold ethical standards in research becomes ever more pressing. The future of medical research ethics depends on a renewed commitment to safeguarding participant rights and ensuring that clinical trials are conducted safely and responsibly. An emphasis on collaboration between institutions, researchers, and regulatory bodies can help in navigating these challenges while maintaining high ethical standards.
To effectively address the ethical dilemmas posed by evolving funding landscapes, it is crucial to advocate for protective measures that prioritize participant safety and ethical practice. Exploring alternative funding sources, enhanced transparency, and improved communication between stakeholders can contribute to fortifying medical research ethics in the face of adversity. Ultimately, addressing these challenges is essential for not only advancing scientific knowledge but also for restoring public trust in the medical research enterprise.
Collaborating for Effective Research Practices
Collaboration among hospitals, universities, and research institutions is essential to advancing medical knowledge while ensuring participant safety. Effective communication and coordinated efforts allow for the sharing of best practices, resources, and strategies in addressing ethical concerns. Through collaborative frameworks, IRBs can consolidate their review processes, streamline approvals, and foster a culture of accountability that prioritizes participant protection and safety.
However, collaboration is contingent on the availability of adequate funding to support these partnerships. As federal research grants face cuts, the innovation and effectiveness of collaborative research efforts are at risk. Ensuring that institutions work together towards a common goal of safeguarding participants also requires securing financial commitments that uphold the necessary oversight of clinical trials. The shared responsibility for promoting patient safety rests on the entire ecosystem of medical research and its ability to sustain collaborative practices.
The Consequences of Research Suspension: A Broader Perspective
Suspensions in medical research due to funding cuts have far-reaching implications that extend beyond merely halting a study. These disruptions can lead to delays in potentially breakthrough therapies, impacting not only participants but also the larger population that would benefit from innovative treatments. The moral obligation of researchers to their study participants and society as a whole underscores the pressing need for continuous support in medical research efforts.
Moreover, these funding interruptions can erode confidence in the medical research system, potentially discouraging future participation in clinical trials. Participants who may have previously volunteered for studies out of hope for improved treatments might hesitate in light of funding cuts and suspensions, fearing for their safety and wellbeing. To counter these negative perceptions, it is imperative for the research community to highlight the safeguards and ethical frameworks in place that protect participants even amidst financial challenges.
Building a Sustainable Framework for Medical Research
Creating a sustainable framework for medical research requires a multifaceted approach that addresses funding challenges while enhancing participant protections. Engaging policymakers, research institutions, and the public is essential for developing strategies that prioritize ethical considerations and secure continued support for IRB mechanisms. This collaborative approach can help cultivate a more resilient research environment that not only mitigates the impact of funding cuts but emphasizes the importance of participant safety.
By fostering an understanding of the intricate balance between research funding and ethical oversight, stakeholders can work collectively towards solutions that uphold the standards of medical research. The ongoing dialogue around funding implications, participant safety, and ethical frameworks must remain at the forefront of discussions regarding the future of medical research, ensuring that the rights and wellbeing of participants are never compromised.
Frequently Asked Questions
How does patient safety in medical research get ensured through IRB oversight?
Institutional Review Boards (IRBs) play a crucial role in ensuring patient safety in medical research. They review research proposals to safeguard the rights, welfare, and well-being of participants. By evaluating study designs, informed consent processes, and risk mitigation strategies, IRBs help to ensure that potential harms are minimized and that ethical standards are strictly followed.
What impact do funding cuts have on patient safety in medical research?
Funding cuts can severely impede patient safety in medical research by halting essential oversight functions conducted by IRBs. When grants are canceled or frozen, ongoing research studies may be interrupted, which can lead to increased risks for participants, delayed treatments, and erosion of public trust in clinical trials and research.
Why are institutional review boards important for research participant protection?
Institutional Review Boards (IRBs) are vital for research participant protection as they provide oversight by ensuring that studies comply with ethical standards and regulations. They assess the risks and benefits of medical research, facilitate informed consent, and monitor ongoing trials to maintain patient safety and integrity in the research process.
How do key historical events reflect the importance of patient safety in medical research?
Historical events such as the Tuskegee Study and unethical hepatitis research emphasize the necessity of patient safety in medical research. These incidents led to the establishment of IRB oversight and ethical standards that protect research participants, demonstrating that rigorous ethical oversight is essential to maintaining trust and safeguarding the rights of those involved in studies.
What role does NIH funding play in ensuring patient safety in medical research?
NIH funding supports the ethical conduct and oversight of medical research involving human participants. These funds help enable IRBs to operate effectively, providing necessary reviews and ensuring compliance with safety regulations, which are vital for protecting research participants throughout the research lifecycle.
What challenges do IRBs face that could affect patient safety in clinical trials?
IRBs face numerous challenges, including funding cuts, which can disrupt their ability to conduct thorough reviews, train staff, and ensure compliance with ethical standards. When resources are limited, IRBs may struggle to maintain the rigorous oversight needed to protect patient safety in clinical trials, increasing the risk of potential harm to participants.
How can the structure of multi-site research impact patient safety?
Multi-site research often requires a single IRB (sIRB) to oversee the ethical conduct of studies across various locations. While this can streamline approval processes, ensuring patient safety requires robust communication and adherence to ethical standards across all sites involved. Any lapse in this integration can jeopardize participant protection.
What should participants know about their safety in medical research studies?
Research participants should be informed about the risks, benefits, and procedures of the study in which they are involved. They have the right to ask questions, withdraw from the study at any time, and expect that an IRB is monitoring the protocol to safeguard their rights and safety throughout the research process.
How can community trust in medical research be maintained through patient safety measures?
Building and maintaining community trust in medical research hinges on transparency and ethical practices. Ensuring that studies are overseen by qualified IRBs, providing clear information about participant rights, and demonstrating a commitment to patient safety can enhance public confidence in the research process.
| Key Point | Details |
|---|---|
| Funding Cuts Impact Research | The Trump administration’s $2 billion freeze disrupts oversight of multiple-site medical studies, affecting patient safety. |
| Importance of SMART IRB | SMART IRB facilitates collaboration between hospitals, universities, and federal agencies, ensuring patient rights and welfare. |
| Role of IRBs | Institutional Review Boards review and oversee research proposals, ensuring informed consent, risk assessment, and participant safety. |
| Historical Context | Past ethical breaches in research have led to the establishment of IRBs to protect patient rights and ensure ethical conduct. |
| Ongoing Transitions | Halting studies can lead to risks for participants and reinforce public skepticism towards medical research. |
Summary
Patient safety in medical research is a critical issue, particularly in light of recent funding cuts. The significant halt in federal research funding has created notable disruptions in oversight systems vital for protecting participants in medical studies. The role of Institutional Review Boards (IRBs) has become increasingly essential to ensure compliance and safeguard the welfare of research subjects. With a history marked by ethical failures, the importance of adherence to safety protocols cannot be overstated. As more studies are disrupted, the potential risks to patient safety heighten, underscoring the critical need for ongoing support and funding in the medical research field.